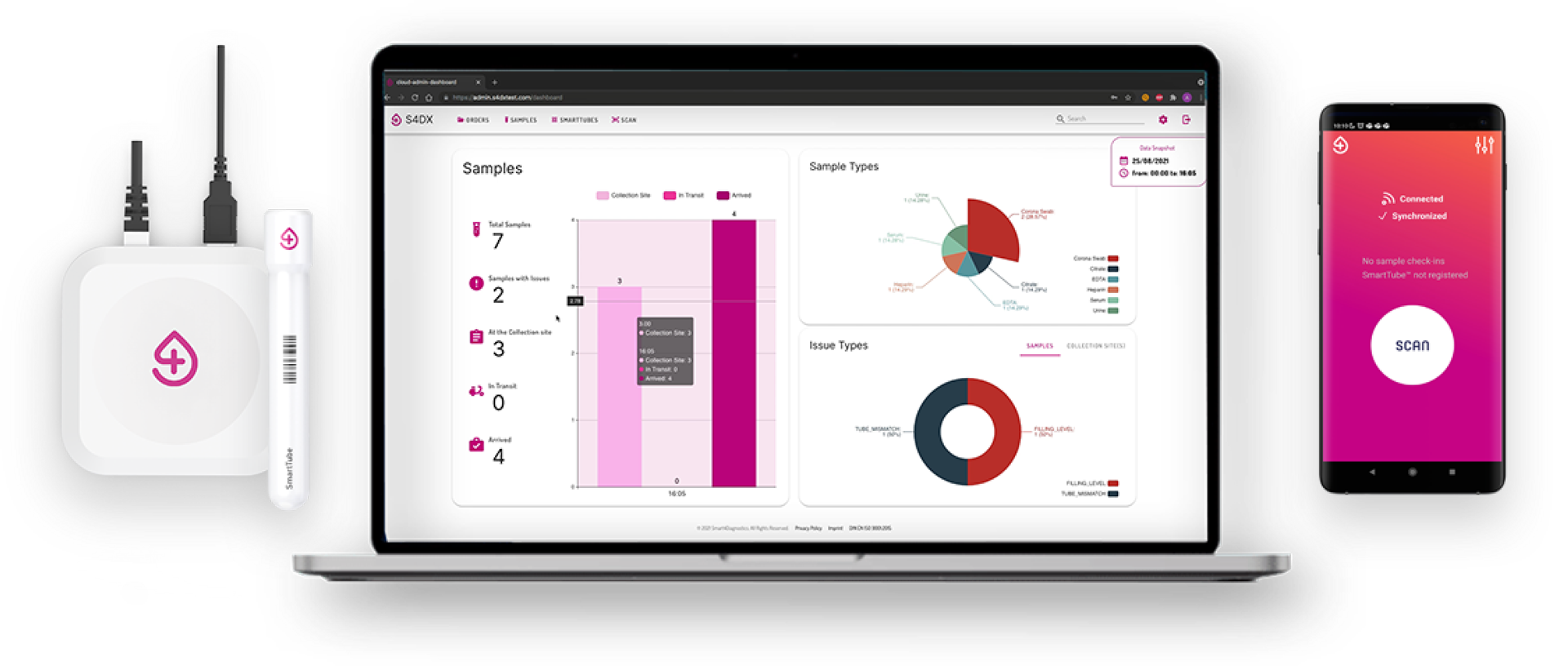
Smart.Safe.SamplesTM
The new quality standard in preanalytics
S4DX provides solutions to every stage of the preanalytics cycle. Fully adaptable and customizable, used individually, in combination or integrated, our range of products bring excellence to the different operations in the diagnostics scope
Frequently asked questions
-
Are there any limitations on sample containers to be processed by S4DX?
No, S4DX can process all standard sample containers (even pre-barcoded) independent of the manufacturer and the container form. S4DX processes blood, tissue, urine, swab samples, and even blood bags.
-
Are there any limitations related to sample transport methods?
No, S4DX adapts to every sample transport method in preanalytics; no matter if you use bags or boxes or pneumatic tube systems; no matter if you drive, walk, ride or fly your samples from collection to laboratory; with S4DX you do not have to change your processes.
-
Are there any limitations because of preanalytical sample handling methods in the laboratory?
No, S4DX adapts to your laboratory routine. No matter if you process samples by hand (manually) or with bulk sorters and other machines. Our system is fully adaptable to all processes.
-
Are there any limitations on lab-hardware to be compatible with S4DX?
No, S4DX operates with all lab hardware and all laboratory machines by using common interfaces.
-
Do I have to change my routine in sample collection?
No, S4DX adapts to your individual sample collection routine.
-
How are transport conditions monitored?
Transport conditions are monitored by the S4DX SmartTube Datalogger that continuously records environmental data (time, temperature and shock events).
-
How is the S4DX SmartTube Datalogger used at the collection site?
S4DX SmartTubes do not need to be activated (e.g. pushing a button). They are registered by being scanned and are digitally linked to all samples that are sent to the lab.
-
How is the S4DX SmartTube Datalogger used at lab arrival?
S4DX SmartTubes do not need to be activated for data extraction. They are automatically recognized at the lab entry by the S4DX Gateway (via Bluetooth). Data from the samples and the transport (time, schock events, temperature curves, any kind of incidence) is then transmitted automatically and there is no need for any kind of manual step at the laboratory entry. This eliminates a lot of manual work (by eliminating for instance the need of scanning samples or boxes at lab entry) and enables for a fully automated and digital lab entry of samples.
-
Is the S4DX SmartTube Datalogger reusable and hygienically safe?
Yes. If used in a room-temperature range, S4DX SmartTube Dataloggers will operate for around two years. S4DX SmartTubes can be disinfected by wipe-disinfection with commonly used disinfectants (incl. alcohol-/formalin-based disinfectants). Autoclavation is not possible.
-
Can the S4DX SmartTube Datalogger be processed by a Bulk Sorter?
Yes, the S4DX SmartTube has conventional blood tube dimensions and can be processed by standard bulk- or sample sorter. The S4DX SmartTube will be sorted out automatically.
-
Is the S4DX SmartTube connected with several collection sites?
Yes, the S4DX SmartTube Datalogger is only linked to the lab and not to a single collection site. S4DX SmartTube Datalogger can travel free within all collection sites of your laboratory.
-
Can I use S4DX for outpatient and inpatient setting?
Yes, S4DX is designed to be used in all settings of human sample collection.
-
What are the main benefits for the collection site?
- Automated timestamps – no manual timekeeping of collection, transport departure or arrival time at laboratory necessary
- Full order verification – digitally matching sample containers with patients and lab orders
- Phlebotomist guidance - individual handling advice for samples before and after collection
- Peace of mind – full assurance if orders are complete in quantity and quality
- Patient safety- link to S4DX preanalytical data in patient results to always ensure results are not related to preanalytical sample incidences -
What are the main benefits with S4DX for the laboratory?
- Less reorders – permanent reduction of preanalytical errors up to 70 %
- Predictive sample workflow - information ahead of time on quantity and quality of samples traveling to the laboratory
- Audit ready data – on the individual sample in preanalytics, matching with ISO 15189 requirements
- Auto reception of samples in laboratory
- Resource optimization at laboratory entry
- auto reception of samples in laboratory
- resource optimization at laboratory entry
-
Which interface protocol do you use?
We use REST-APIs. Interfaces between S4DX and the LIS are only set up once. After this, export and import will be standardized in routine. We prefer bi-directional interfaces for lab order verification. However, also uni-directional interfaces are possible. Additionally, we can support other protocols, e.g. HL7 FHIR.
-
Do I have to set up interfaces with collection sites?
No, S4DX does not need any interface with collection sites.
-
Do I need a specific order entry system to use S4DX?
No, S4DX does not need a specific order entry system or LIS.
-
Not all collection sites are connected to an order-entry system – does S4DX also operate without order entry?
Yes, S4DX can operate without any order entry or LIS interface. This limits full order verification, but all other functionalities are still available.
-
How do you work with sample containers that are labelled with the patient's name?
S4DX is ISO 27001 certified and is able to handle patient information. However, we only scan the barcode and do not make any picture of other information on the tube.
-
How is the patient identified when using S4DX?
Patients can be identified by the scan of a patient wristband or any other patient barcode. We do not process any patient medical or clinical records. Just the patient name and demographics are needed for correct identification.
-
What information do you process on the courier-driver?
Timestamps for sample pick-up, handover and delivery are monitored, as well as temperature, shock-profiles and transport duration. Additionally, the S4DX Courier app can deliver one GPS-location per minute (no GPS recording).
-
Does S4DX provide hardware?
S4DX provides SmartTube Dataloggers and laboratory Gateways. Devices necessary for scanning or running our applications are not provided.